Here is Mr Fattah’s advice and information in plain English for those wondering why some breast implants have been withdrawn. Also, what exactly is Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL) and should I worry about it? Mr Fattah has discussed and provided written information about this with all his patients at consultation. But with the latest development feels this should be shared with anyone considering breast augmentation.
What is the issue?
There is some data to show that a very rare cancer now called Breast Implant-Associated Anaplastic Large Cell Lymphoma is related to the use of textured breast implants. BIA-ALCL is rare and treatable and was first described in 1997. Initial research suggested the risk of developing this condition is approximately 1 in 300,000 implants sold, but newer studies suggest the risk may be higher.1 By contrast, the incidence of breast cancer in the UK is 1 in 10 and affects women the same whether or not they have had breast implants. The risk of death and complications from breast cancer is much higher than for BIA-ALCL.
What is BIA-ALCL?
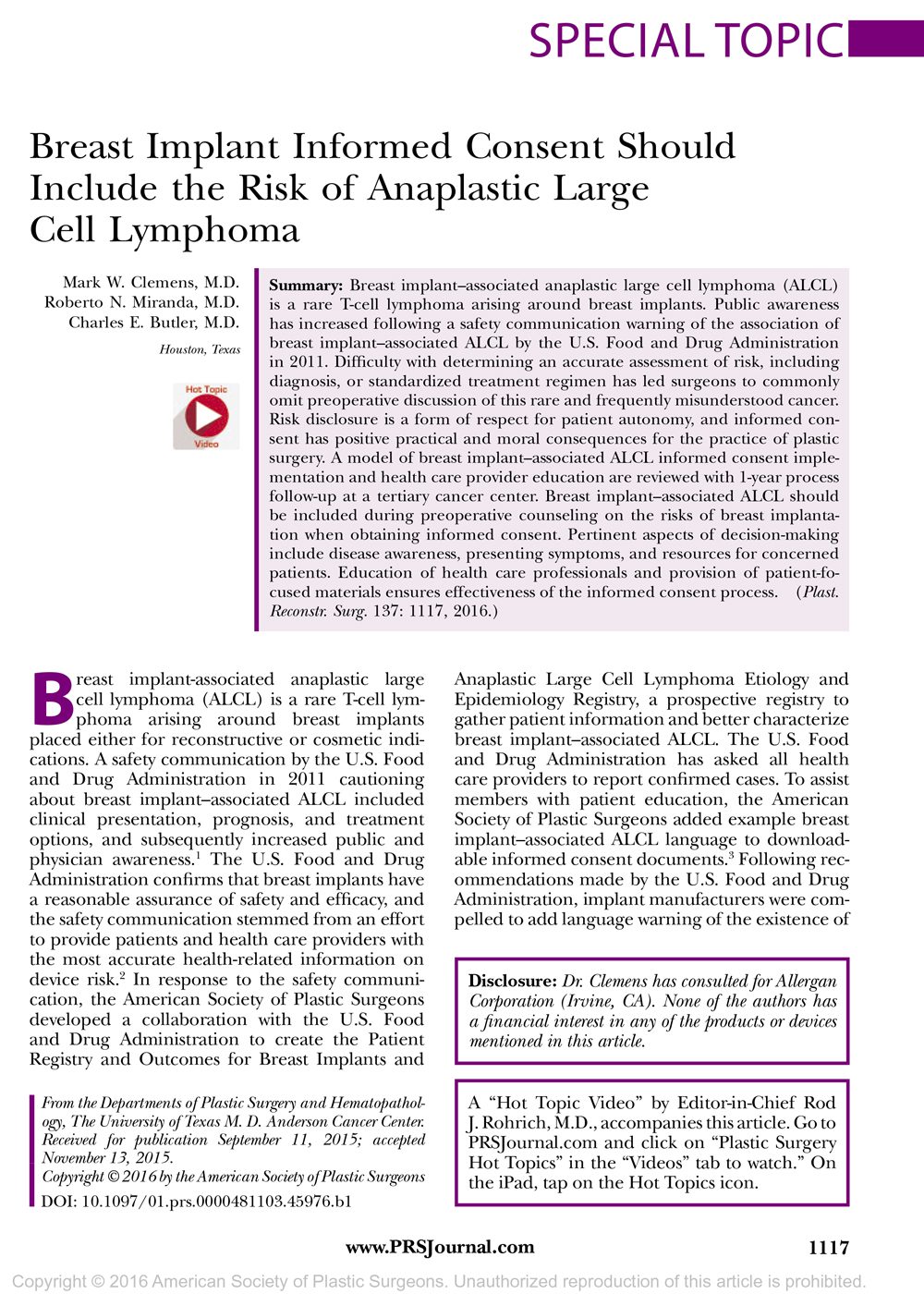
Ok, here’s the science part: The body has arteries (take blood from the heart) and veins (return blood to the heart) but this system is leaky and so the body has an overflow system of pipes termed the lymphatic system. The lymph “glands” (eg. in your neck when you get a cold) are actually filters in this system that trap bacteria or other unwanted cells and direct the body’s defenses to kill them there (which is why they swell up as the body’s white cells gather at the battlefield to kill the invaders!). The cells that comprise the lymph system can occur all around the body and can gather wherever they need to fight abnormal cells. A lymphoma is a cancer of any part of this system, anywhere in the body. Non-Hodgkins Lymphomas make up 90% https://www.cancer.org.au/about-cancer/types-of-cancer/lymphoma.html of these and BIA-ALCL is a member of this group. BIA-ALCL occurs as a thickening of the breast implant capsule – this is the otherwise normal layer of tissue that forms around the breast implant. Only in the later stages of the disease would it spread to the lymph glands or elsewhere in the body.
How would I know if I had it?
Ordinarily, you wouldn’t feel such a thickening; the most common presentation is a swelling of the breast with fluid (a seroma) more than one year after insertion of the breast implants. This happens to 1-2 per 1000 of women without any cause anyway. Furthermore, most cases of a seroma occur following an infection or a history of trauma (such as lifting or pushing a heavy weight creating some discomfort followed by breast swelling over the following day or so). Other features described include skin rash, capsular contracture (see below) and swollen glands in the armpit (axillary lymphadenopathy): but of course, all of these have more common causes than BIA-ALCL.
What would happen if I thought I had this?
There is no need to panic and you should continue with your recommended follow up – we know that ultrasound is the best way to accurately detect and confirm a seroma and we recommend an ultrasound if an unexplained swelling occurs in your breast. At this ultrasound, if possible, a small (20ml) sample of the fluid will be taken and sent to the laboratory. In addition, the ultrasound can look for any lumps and any found would need to be biopsied. If there was any further suspicion then further tests (such as PET scans) may be performed before treatment is considered. These are to look for the risk of the disease having spread away from the implant capsule.
What is the prognosis?
If the disease is picked up early (just a seroma) where the tumour is confined to the capsule (86.2% of cases)2, then it has a relatively benign course and is very treatable. In the remaining cases, there may be a need for chemotherapy before surgery to shrink the disease or after surgery to get rid of any remaining disease. The key point is that early detection allows for surgical removal without the need for additional treatment. A total of 16 patients have died from the disease worldwide – the main issue being a significant delay in diagnosis and treatment.

How is it treated?
The removal of the implant with the capsule would be the key and complete removal of early stage disease is virtually curative.2 Breast reconstruction can be performed and replacement with smooth implants may be considered as these do not appear to be related to the condition: but the decision of what to do next would be firmly in your hands as the patient. You will not need a mastectomy or full lymph gland removal for this condition. It is very different disease and treated in a very different way to breast cancer.
Why does it only occur in textured implants?
We don’t know, but the current theory is that the textured surface of some implants creates a chronic inflammation that in the genetically susceptible person can lead to the development of this condition.
Why do we use textured implants?
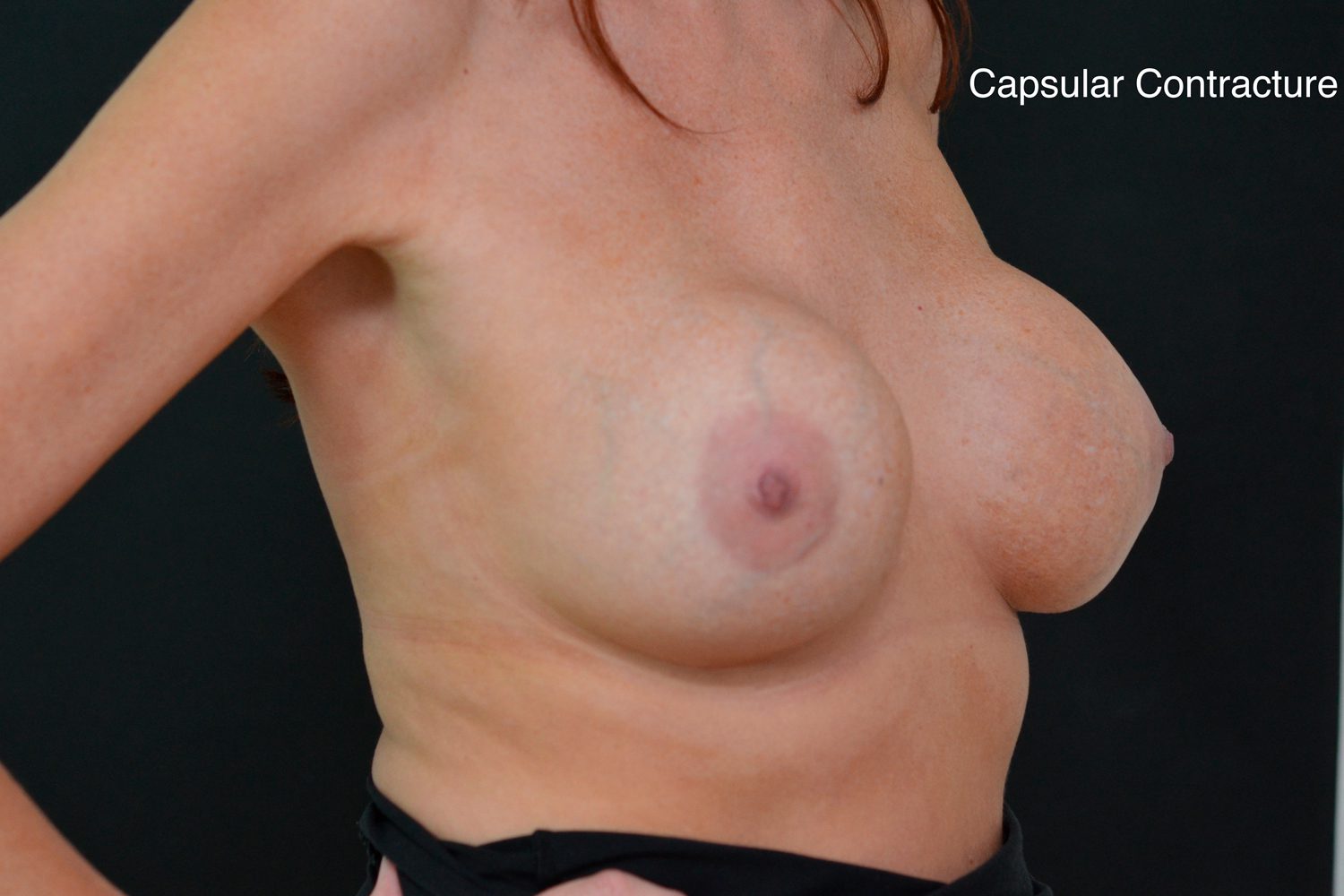
Not all textured surfaces are manufactured in the same way and they appear to convey different levels of risk, hence it is difficult to draw definite conclusions at this time. Texturing of an implant surface also offers advantages – the main one being a reduction in the risk of capsular contracture. Capsular contracture is recognised by surgeons as being the number-one cause of problems for patients.3 Contracture is when the capsule starts to tighten and squeeze the implant making it feel hard, look abnormal or even become painful in late stages. Capsular contracture may require further surgery so this is why surgeons are keen to use implants that minimize this issue.
Why have Allergan textured implants been withdrawn?
Several different companies manufacture breast implants for both cosmetic and reconstructive use. These implants can have different types of texturing on their surface and some research has indicated that BIA-ALCL might be related to a particular type of texturing. One major company, Allergan, produces implants known as Natrelle® with a surface called Biocell®. These implants are available worldwide but can only be sold under licence (the CE mark).
All manufacturers are periodically required to apply for re-certification of their CE mark to continue to supply their devices for patient use. Due to concerns over the possible link with Allergan’s textured surface (Biocell®), the CE mark has not been re-awarded for this product. Hence from late December 2018, Allergan’s textured implants are no longer available for patient use. This is a precautionary measure until more data becomes available and Allergan continues to work with the authorities to clarify the issue. This is partly due to timing (Allergan’s licence was up for renewal anyway) and that fact that data from other manufacturers was not as clear.
What prompted this decision?
Recent publications in the scientific literature have looked at multiple studies including nearly 100,000 patients in an American database to look for any issues regarding the use of breast implants. Additionally, other studies were combined together to look for rare issues. As a result of this, there appears to have been a link between textured implants and BIA-ALCL – mainly those with the Allergan Biocell® coating. The studies do have some limitations – for example different manufacturers’ implants could not be directly compared and that many symptoms were reported by patients but not confirmed by a physician. However, there was enough concern for the French regulator (ANSM) to recommend not renewing Allergan’s CE licence. The failure to renew the CE mark for Allergan’s textured implants does not at this stage affect any other manufacturer’s texture implants or Allergan’s smooth-surface breast implants. There is nothing that we can find on Allergan’s website or the Natrelle® website that refers to this issue at the moment (December 21st).

What does this mean if I have had Allergan implants?
The ANSM have stated in their own publication that there is no immediate threat to the health of women with textured Allergan implants but that all women with breast implants should be reviewed annually (Mr Fattah’s routine practice). The MHRA (UK regulator) https://www.gov.uk/government/news/mhra-statement-on-allergan states there is currently no evidence of an increased risk to patients. No regulator in the world has yet suggested that implants should be removed.
What should I do now?
Nothing needs to be done now. Mr Fattah has not routinely used Natrelle® implants; If you are a patient of Mr Fattah, you will receive a letter outlining the above. If you are a past or current patient and you wish to discuss this further please don’t hesitate to contact the clinic where you saw Mr Fattah and arrange an appointment. If you are not sure what manufacturer or implant you have just drop us a line and we can provide you with the a copy of your operation note that will record the implants make, model and serial number. Remember is this is a precaution because we are not sure what the links are rather than a reaction to a proven issue. Additionally, your risk of a poorer-prognosis breast cancer is much higher that BIA-ALCL and this is not affected by having breast implants. Feel free to contact us for more information and reassurance.
References
1. Loch-Wilkinson A, Beath KJ, Knight RJW, et al. Breast Implant–Associated Anaplastic Large Cell Lymphoma in Australia and New Zealand. Plast Reconstr Surg. 2017;140(4):645–654. doi:10.1097/PRS.0000000000003654.
2. Clemens MW, Brody GS, Mahabir RC, Miranda RN. How to Diagnose and Treat Breast Implant-Associated Anaplastic Large Cell Lymphoma. Plast Reconstr Surg. 2018;141(4):586e–599e. doi:10.1097/PRS.0000000000004262.
3. Coroneos CJ, Selber JC, Offodile AC II, Butler CE, Clemens MW. US FDA Breast Implant Postapproval Studies. Ann Surg. 2019;269(1):30–36. doi:10.1097/SLA.0000000000002990.
